Tepmetko may be a valuable drug option for patients with metastatic NSCLC with MET exon 14 skipping alterations.
While the Food and Drug Administration’s (FDA) full approval of Tepmetko (tepotinib) for metastatic lung cancer proves “validation” that this drug is a valuable option for this patient population, there are still quality-of-life issues to consider, explained Dr. Paul K. Paik, clinical director of thoracic oncology services at Memorial Sloan Kettering Cancer Center.
In February 2024, the FDA granted a full approval to Tepmetko for patients with metastatic non-small cell lung cancer (NSCLC) with MET exon 14 skipping alterations. The approval is coming a 2021 accelerated approval for the same indication.
Full Approval Validates Findings, May Broaden Use
“What is particularly satisfying — and I think relevant for patients — is that not only has the updated data that led to the approval validated the findings, but really, with additional patients who are treated and additional time on the study, it has shown that the efficacy has really gone up, compared to when the data was first reported in 2020,” Paik said in an interview with CURE®.
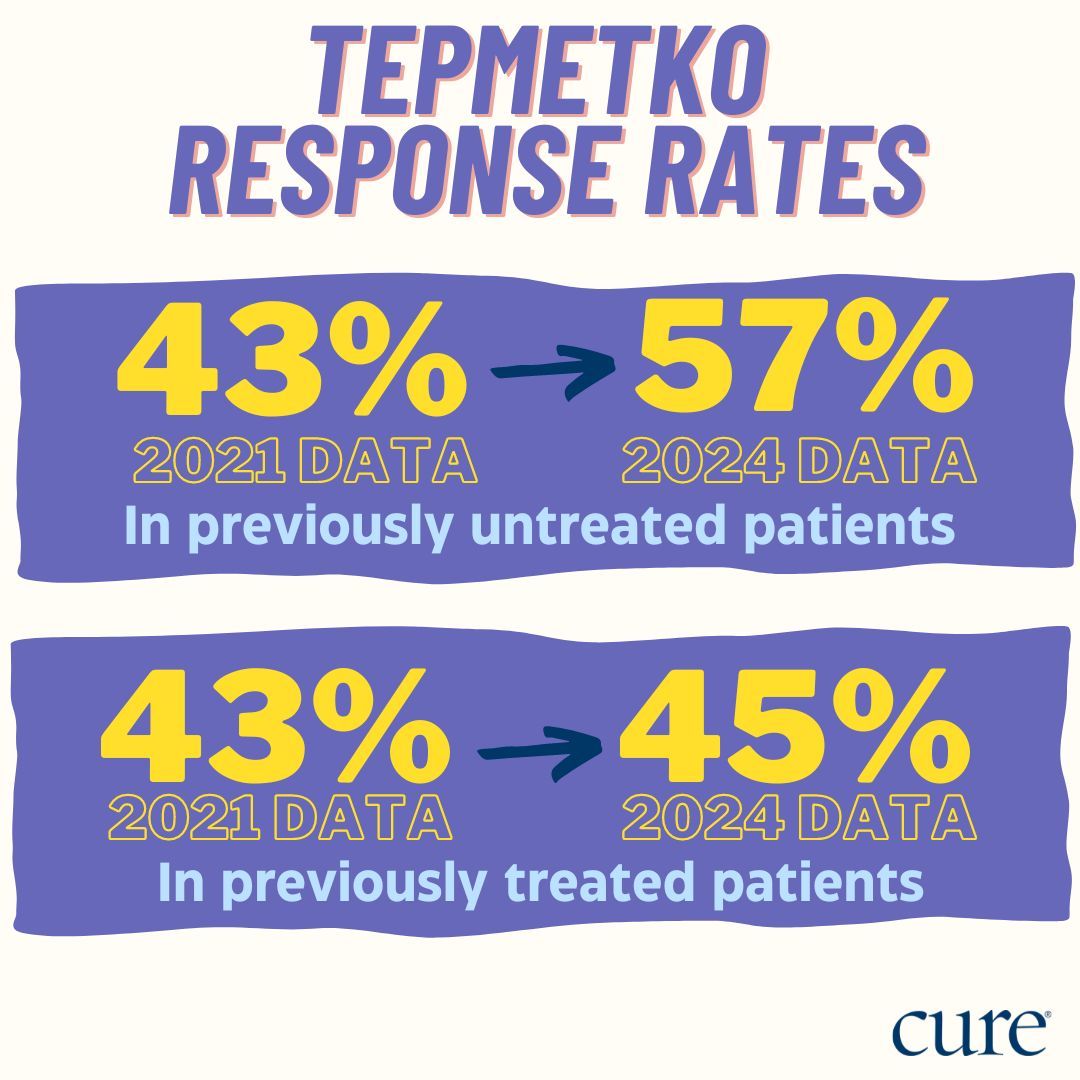
Specifically, in patients who were pretreated, the data leading to the 2021 accelerated approval showed that the overall response rate (ORR; percentage of patients whose disease shrunk or disappeared from treatment) was 43%. Later, the 2024 data showed that the ORR increased to 57%. For patients who received prior treatment before Tepmetko, the ORR was 43% and 45% in the 2021 and 2024 datasets, respectively.
The goal of the accelerated approval was to get the drug more quickly into the hands of patients who need it. Now, Paik hopes that the full approval will expand Tepmetko’s use in the future.
Shown are the overall response rates for patients with a specific lung cancer subset receiving Tepmetko.
“The hope with the full approval and the updated data is that providers — and particularly those out in the community [setting] — will be able to take a look at this and see that it is a very good and reasonable therapy for our patient population of [individuals with] MET exon 14 skipping-positive lung cancer,” Paik said.
Managing Swelling from Tepmetko
Paik mentioned that Tepmetko is a “fairly well-tolerated drug,” that is taken orally (meaning that patients can take it anywhere and do not need to come to the clinic) there are some side effects, such as peripheral edema, that can impact patient’s quality of life.
Peripheral edema is swelling of the arms or legs that results from fluid retention and happens to more than half of patients taking Tepmetko, according to Paik.
“The swelling itself is benign; it doesn’t really cause any other complications,” Paik said. “But it is a major quality of life issue for some patients — particularly for patients who are older where mobility is already impaired.”
Paik mentioned that, unlike other side effects, swelling does not happen soon after treatment. In fact, the average time to onset, he noted, is approximately two months. That said, it is crucial that patients continue to follow up with their health care teams to address any issues or side effects that they encounter.
Treatment of peripheral edema involves redistributing the fluid to other parts of the body. Sometimes, patients may temporarily stop Tepmetko and wait until the swelling goes down before starting the therapy again. Patients may also seek help from lymphedema specialists, which are clinicians who specialize in handling edemas.
Next Steps for Tepmetko and Lung Cancer
Paik mentioned that ongoing research is analyzing why side effects such as edema happen, and how they can be prevented or handled to ensure that patients have the best possible quality of life.
Additionally, he hopes to see answers as to why certain patients experience progression on Tepmetko, and what can be done to improve outcomes for this patient population.
“It’s already a pretty effective drug, but most patients will end up developing disease progression at some point — just like all of our cancer therapies — on this medication,” he said. “So understanding why that happens and how to improve and circumvent that resistance is really going to be the focus moving forward.”
For more news on cancer updates, research and education, don’t forget to subscribe to CURE®’s newsletters here.






